A Review of Catalysts for the Electroreduction of Carbon Dioxide to Produce Low-carbon Fuels
Introduction
Climate change, of which greenhouse gas emission is the main driver, is one of the near urgent challenges humanity is currently facing. Equally depicted in Figure 1, the atmospheric COtwo concentration has been rising rapidly since the showtime of the measurements in March 1958, with an average increase of approximately 2 ppm per year over the by decade (Tans, 2019). In 2016, the atmospheric CO2 concentration stayed above the symbolic 400 ppm marker all year round for the showtime time, corresponding to a 30% increment compared to the pre-industrial (before circa 1750) levels of 270 ppm (Betts et al., 2016). The Paris Agreement under the Un Framework Convention on Climatic change adopted in 2015 illustrates the worldwide commitment to reduce greenhouse gas emissions to mitigate global warming, only this will crave an order-of-magnitude increment in public and private investments in inquiry and development between 2019 and 2030 (Rockström et al., 2017).
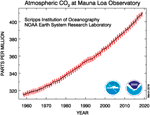
Figure 1. Concentrations of CO2 in the atmosphere measured at Mauna Loa Observatory (Hawaii) since the offset of the measurements in March 1958 (316 ppm) (Tans, 2019).
Strategies to reduce CO2 emissions can be divided in 4 categories that focus on either avoidance of COii emissions or bounden the emitted CO2 in a natural or non-natural sink. The start category is improving free energy efficiency, which currently provides the greatest return on investment and has already been successfully practical in many industrial contexts. Although this approach yet has potential, annual improvements of 1–2% will not be sufficient to meet the climate targets. The second category, using non- or depression-carbon energy sources, (e.g., solar, current of air, geothermal), is at big scale still challenging due to the fluctuating nature of the energy supply and the tiresome rate at which the electricity production is becoming more renewable. Carbon Capture and Sequestration (CCS), i.east., a series of technologies combining CO2 capture from large point sources such as power plants, transportation to a storage site, and sequestration into a (natural) sink, is the tertiary category, but its potential is currently rather limited due to technical and economic hurdles (Spigarelli and Kawatra, 2013; Leung et al., 2014). The fourth category is Carbon Capture and Utilization (CCU), in which CO2 is converted to (loftier-value) products. This category can be considered every bit a special example of the third category with the utilization part acting as a non-natural sink (Whipple and Kenis, 2010; Kuhl et al., 2014; Schouten et al., 2014).
CO2 is a thermodynamically very stable molecule and thus a substantial input of energy combined with constructive reaction conditions and active catalysts are required for its conversion, c.f. Figure 2. To obtain the desired overall negative COii balance, the energy required for its conversion should originate from non- or depression carbon free energy sources. Hence, the development of COii conversion processes has focused on minimizing the required energy input by using the non or low-carbon energy sources in the most efficient way possible. According to a recent report (Voltachem, 2016), the development of new products through the awarding of innovative technologies powered by renewable energy is one of the main drivers for "electrification" of the chemical manufacture, i.e., replacing thermal and chemical free energy by electric energy. Other main drivers are economic benefits and improved sustainability through the reduction of feedstocks, past-products, waste, energy utilize, solvents, and CO2.

Effigy 2. Standard Gibbs costless energy of formation (at 298 K), expressed in kJ mol−1, of COii and possible reduction products.
Among all the proposed methods for converting COii, which accept as common advantage the ease of integration of non- or low carbon free energy sources, electrochemical methods are considered to exist the almost promising (Endrődi et al., 2017), as several advantages have been claimed compared to the other methods: 1) they can be conducted at ambient conditions (allowing for rapid changes in the product rate as the availability of the renewable energy changes), 2) past a conscientious selection of the electrocatalyst, electrolyte and operating conditions, it is possible to drive the electrochemical conversion of COtwo toward the desired products, 3) the chemic consumption can be minimized by recycling the electrolytes, 4) the reaction systems are compact, modular and hence scale-upward is relatively straightforward, and 5) the electrons are directly used for production formation. Still, there are articulate challenges for electrification, such equally the overall loftier price of electricity, the big investment costs, the often poor selectivities and depression conversions related to low reaction rates (resulting in large reactor volumes needed for a globe-scale plant), the technical and economic feasibility of turning plants on and off safely on brusk find, etc. This implies that at that place is a lot of skepticism whether electrification of the chemical industry is really feasible (Van Geem et al., 2019; Gani et al., 2020) or whether it is another hype similar the numerous ones that take been presented in the final ii decades (Banholzer, 2012; Banholzer and Jones, 2013).
The goal of this report is to explore whether the electrochemical conversion of CO2 tin exist a viable culling product route of ethylene, which is the key building block of the chemic industry and representative for products with a reasonably high added value. First, a curt overview is given of the CO2 reduction process and the performance trends with the focus on ethylene formation. Next, a techno-economic model is developed for a COtwo conversion plant integrated with CO2 capture from a blast-furnace flue gas stream. With this model, the economic competitiveness of this alternative ethylene product route is compared against the current state of the art for ethylene production, i.e., naphtha-based steam cracking, under both electric current and future conditions. Finally, the CO2 avoidance potential of the process is assessed based on a Life Cycle Analysis, adopting a cradle-to-gate boundary.
Methodology
Electrochemical Conversion of CO2
The electrochemical reduction of CO2 is a complex conversion consisting of multiple elementary proton-electron reactions leading to the (co-)germination of various products of which ethylene has the highest commercial value. Equally depicted in Figure 3, CO2 is converted at the negatively charged cathode to primarily CO, methane, ethylene and formic acid, while HiiO is oxidized into O2 at the anode. The half-cell reactions for the electrochemical reduction of CO2 and the corresponding formal reduction potential are summarized in Table 1 (Bard et al., 1985). For all possible reduction products, the reaction proceeds via CO equally intermediate species (Hori, 2008). In aqueous environment, the hydrogen evolution reaction competes with the reduction of CO2. Aside from the employed electrocatalyst and electrode potential, the product distribution obtained from the electrochemical reduction of CO2 depends on the choice of electrolyte, the electrolyte concentration, the concentration of dissolved CO2 and the reaction conditions, i.east., pressure and temperature.
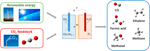
Figure 3. Schematic representation of the product of high-value chemicals past the electrochemical reduction of COii making utilize of renewable energy sources.
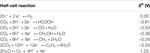
Table 1. Half-prison cell reactions and the corresponding formal redox potential E 0 (Five) for the electrochemical reduction of COtwo. All potentials are referenced confronting the standard hydrogen electrode (Bard et al., 1985).
To evaluate the technological operation of this electrochemical process and enable a meaningful comparing between different electrocatalysts, several figures of merit are commonly used (Pander Three et al., 2017). Because the reduction of CO2 has to overcome a kinetic energy barrier, the cell potential at which the redox reaction is experimentally observed (Due east), is college than the reversible jail cell potential (E 0) and the departure betwixt the two is denoted every bit the overpotential
Minimizing the overpotential of the desired electrochemical reaction minimizes the required energy input. The Faradaic efficiency
With
In general terms, the lower the overpotential and the college the Faradaic efficiency, the college the energy efficiency of the process. Finally, the current density
Procedure Weather condition and Selectivity
To enable a viable large-scale implementation of an electrochemical CO2 reduction process, the development of an agile, selective, stable, and relatively low-cost electrocatalyst is a prerequisite. Over the last few years, many researchers focused on the exploration of different electrocatalysts with the aim of addressing the cardinal challenges of this electrochemical process (Hori et al., 1985; Hara et al., 1997; Hori, 2008; Rakowski Dubois and Dubois, 2009; Peterson and Nørskov, 2012; Schneider et al., 2012; Qiao et al., 2014; Kortlever et al., 2015; Mao and Hatton, 2015; Engelbrecht et al., 2016; Kortlever et al., 2016; Wu et al., 2016; Tao et al., 2017): 1) reduce the overpotential (or increase energy efficiency), ii) increase the selectivity (or Faradaic efficiency), 3) increase electric current density, and iv) expand catalyst lifetime (less than 100 h) with order of magnitude. While new studies reporting improved Faradaic efficiencies and lower overpotentials are consistently being published, the of import question remains what the operation of the reduction process should be to enable implementation of a viable large-scale industrial process. As a dominion of pollex, industrial reactors are typically operated at geometric current densities in a higher place 100 mA cm−2 with at least 50% Faradaic efficiency for the required products, in order to minimize investment costs as much as possible (Oloman and Li, 2008). In Figure 4, an overview is given of the electrochemical performances for CO2 reduction to ethylene from a selection of studies reported in the open literature published in the period from 1986 to 2017. The overpotential, which determines the free energy efficiency of the procedure, ranges from −0.8 to −2.iv V. While meaning progress has been made over the last few years, the functioning of state-of-the fine art technologies seems to be currently not yet at the level required for an economically viable large-calibration process, indicated past the light-green zone and applying the dominion of pollex specified higher up.
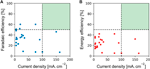
FIGURE 4. Summary of the electrochemical performance of CO2 reduction to ethylene from a selection of studies reported in the open literature published in the period from 1986 to 2019: Faradaic efficiencies (A) and free energy efficiencies (B) equally a function of current density (mA cm−two) (Hori et al., 1985; Kaneco et al., 1999; Ogura et al., 2004; Hori, 2008; Engelbrecht et al., 2016; Ma et al., 2016; Mistry et al., 2016; Wu et al., 2016; Ke et al., 2017; Peng et al., 2017; Gao et al., 2019).
Aside from activity and selectivity issues, rapid deactivation of the catalysts, which leads to a shift in the product distribution favoring the hydrogen evolution reaction, is also one of the chief challenges. In most cases, the goad lifetime is under 100 h (Qiao et al., 2014). The factors influencing the catalyst lifetime have so far not been analyzed in item, also because almost experiments are performed in a limited time span focusing on improving the initial catalyst performance. Although the verbal cause for catalyst deactivation is not ever articulate, several hypotheses have been suggested, including electrolyte trace impurity degradation, accumulation of adsorbed or insoluble reaction by-products and morphological changes of the catalyst. A lifetime in the society of magnitude of thousands of hours is required for a viable large-scale process. Otherwise, frequent regeneration of the electrodes should be foreseen in the process (Dominguez-Ramos et al., 2015; Martin et al., 2015).
It is clear that several technological breakthroughs are needed before the electrochemical reduction of CO2 can go industrially feasible. A roadmap for the electrochemical reduction of CO2 has recently been developed within European union'southward Energy program, proposing both brusque-term and long-term practical goals (Koper and Roldan, 2019). In the adjacent 5 years, pregnant progress should be made on the development of COtwo electrocatalysts and electrolyzers, operating at relevant current densities (>100 mA cm−2), with high Faradaic efficiency for high-value products such as ethylene, at lower overpotential (2.0–2.2 V), and skilful stability (>100 h). In the long term, the integration with downstream operations too every bit integration with upstream CO2 capture should be considered.
Conceptual Process Design
In Effigy five, a conceptual process scheme for the electrocatalytic conversion of CO2 is depicted. The first step of the procedure is the capture of CO2 from the flue gas stream and send to the conversion unit. The CO2 source for this study is considered to be blast furnace gas, which contains ∼22 mol% CO, ∼22 mol% COtwo, ∼5 mol% Htwo, and ∼51 mol% Northwardii. The CO2 is captured via chemical absorption with monoethanolamine (MEA) as solvent. The blast furnace technology will go on to dominate steel product in the coming decade and the only fashion to substantially reduce the associated COii emissions is to combine it with CCS or CCU options. In an cushion column the CO2-containing gas stream is contacted with a solvent, afterwards which information technology is desorbed over again from the solvent in a stripper column. As MEA can undergo degradation and is besides lost via the gasses that are vented into the temper, a brand-up of this chemical is required. Chemic absorption with MEA results in a high CO2 purity production stream (>98 wt%), with H2O every bit the primary impurity, while traces of Northward2 and MEA tin can also be present (Li et al., 2016). Gaseous impurities tin have an effect on the electrolysis process in different ways, i.e., they can act as 1) every bit diluents (e.g., Due northtwo), 2) as reducible species (e.one thousand., O2), and 3) as goad poisoning species (e.yard., NO2, SOii, H2S, organic gases) (Zhai et al., 2019). To enable industrial application, the influence of gaseous impurities on the electrolysis process needs to be better understood to avert catalyst degradation. The captured COtwo is combined with a possible recycle stream and sent to the reactor in which the electrochemical reduction takes place. At the cathode, COtwo is reduced resulting in the germination of the main products CO, ethylene, methane, hydrogen and formic acrid, while at the anode H2O is oxidized into Otwo. The global reaction for the production of ethylene is:
The production stream that leaves the cathode compartment of the reactor is sent to a wink vessel. The obtained liquid stream contains mainly electrolyte and unconverted CO2 and can be sent back to the reactor. In gild to avoid the aggregating of liquid byproducts (i.due east., formic acid), part of this stream is purged. The gas stream contains ethylene, unconverted CO2, and significant amounts of other byproducts, such as CO and H2. A gas purification department is required which serves 2 main goals: separation of the unconverted CO2 for re-apply and purification of the desired products (in this example ethylene), and byproducts.
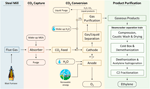
Effigy 5. Envisioned process scheme for the electrochemical conversion of a CO2-containing flue gas stream to high-value chemicals.
Economic Assay
To appraise the actual economic feasibility of this alternative product route for ethylene, the CAPEX and OPEX accept been estimated. A grass-roots institute is considered, congenital in Northwestern Europe. Cost calculations are thus based on European prices. Price functions are introduced to approximate the CAPEX and OPEX for the dissimilar steps of the electrochemical conversion process, i.e., COtwo capture, COii conversion and product separation and purification. Because of the large calibration of this industrial process, the feasibility study is washed for the replacement of a office of the installed product capacity based on fossil fuels, i.e., steam cracking of naphtha. This means it is assumed that the gaseous product stream, after CO2 removal, is farther processed on the separation department of an existing steam corking facility. No costs accept thus been estimated for the product separation and purification steps, except for capturing and recycling of the unconverted CO2. Using estimates from literature data for the specific capital cost and energy requirement, the CAPEX and OPEX are written as a function of four unlike parameters for the dissimilar steps. These parameters, which are related to both the process and to external factors, are: (carbon-based) production selectivity, (single-pass) conversion, CO2 value, and electricity toll. All other parameters actualization in the cost functions are rewritten every bit a function of these 4 disquisitional parameters.
Capital Expenditure Estimation
The estimated CAPEX for the CO2 capture plant is based on a specific capital toll of 70 €/metric ton CO2/twelvemonth (Kuramochi et al., 2011). For the installed cost of the electrochemical reactors, a value of 66 million € is reported in literature, for the conversion of 100 metric ton COtwo per day (Oloman and Li, 2008). This value corresponds to 70 electrochemical menses reactors each with 100 cells of 0.five grand2. No economies of scale are taken into business relationship for the electrochemical reduction of CO2, due to the modular grapheme of the electrochemical cells. To improve the overall conversion of the electrochemical process, unconverted CO2 is separated from the gaseous products and recycled back to the reactor. This is done via an boosted MEA absorption system, for which the same specific capital toll of 70 €/metric ton COii/yr is taken (Kuramochi et al., 2011). Because the assumption is made that the gaseous production stream, after CO2 removal, is further candy in the separation section of the existing steam keen facility, no costs are associated to further production separation and purification steps. The maintenance cost is estimated as ii.five% of the CAPEX.
Operational Expenditure Estimation
The primary energy contribution for the CO2 capture procedure is the generation of steam required for the desorption procedure. This energy is provided making use of natural gas with an efficiency of 85% and a thermal energy price of eight.3 €/GJ (Eurostat (2019)). Mechanical energy required for the pumps and compressors, is causeless to be delivered past electromotors. The thermal and electrical energy requirements for the MEA assimilation organisation are taken from Kuramochi et al. (2011), i.e., three.2 and 0.50 GJ/metric ton CO2, respectively. The cost of MEA losses is assumed to be 4.vi €/metric ton COii (Karl et al., 2011). With respect to the OPEX of the electrochemical reactor, at that place are 2 main contributions: the usage of chemicals and the consumption of free energy (i.e., electricity). The free energy required for the conversion of COii is amidst others determined by the selectivity or equivalently the Faradaic efficiency of the desired reaction, in this case the conversion to ethylene. For a Faradaic efficiency of 60%, approximately 20 MW h per metric ton of converted CO2 is required. The specific energy consumption decreases with a factor two when considering the limiting example of a Faradaic efficiency of 100% (Agarwal et al., 2011). This confirms the importance of technological advancements regarding the energy efficiency of the procedure to enable large-scale industrial production. With respect to the cost of chemicals, the critical assumption is made that the electrolytes tin be fully recycled, which means that the main cost is included in the CAPEX, and in the OPEX only recycling costs need to be taken into account.
Economic Analysis
For the example written report, an almanac ethylene production of ten5 metric ton is considered, which corresponds to approximately 5–20% of a typical ethylene production site based on fossil feedstocks. At 100% conversion and selectivity, the electrocatalytic conversion of CO2 would crave 3.14 × ten5 metric ton of CO2 per year, which corresponds to virtually 5–10% of the CO2 emissions of that aforementioned ethylene production site. Equally a base case, the production of polymer-grade ethylene based on a fossil feedstock, i.e., steam peachy of naphtha, is taken, as this is and volition remain the predominant process for the production of olefins in the coming decades (Amghizar et al., 2017). For this process, a yield of loftier-value chemicals (HVC) of 55 wt% is causeless, with an ethylene and propylene yield of, respectively, 30 and 15 wt% (after hydrogenation of acetylene, methyl acetylene and propadiene). The detailed effluent composition obtained from Zimmermann and Walzl (2000) can exist found in the Supplementary Cloth.
The estimated CAPEX and OPEX for the furnace section of a naphtha-based steam cracker located in Europe are equal to respectively 500 and 225 €/metric ton ethylene (Brown, 2019, Personal Advice). This corresponds to an energy requirement of approximately ix GJ/metric ton HVC or 16 GJ/metric ton ethylene for the furnace section, in agreement with the value reported by Ren et al. (2008). Process upsets, technical issues, and turnarounds, are accounted for via the plant annual uptime, which is equal to viii,440 h per year or 96.3%. An overview of the chief techno economical assumptions can be found in the Supplementary Cloth.
The revenues for the electrochemical process result from the sale of ethylene. Note that high-purity O2 (90–95 wt%) can be considered as a valuable byproduct from the electrolysis process, simply is it not taken into account in the product revenues. If possible, O2 will be used in nearby chemical plants to avert the high-cost send needs. For the conventional steam cracking process the sale of other important products such as propylene, butadiene and BTX is likewise considered. The prices determined for March 2018, are summarized in Table 2, i.e., ethylene at 1,050 €/metric ton, propylene at 840 €/metric ton and naphtha at 500 €/metric ton. The gross margin is calculated every bit the divergence betwixt the revenue from the sale of products and the feedstock price.
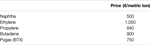
TABLE 2. Applied pricing (March 2018) level for the major materials in this study (ICIS Pricing Database; Platts Global Ethylene Price Index; Platts Global Propylene Price Alphabetize).
5 hypothetical cases were considered to appraise the economic potential of this alternative ethylene product route compared to the base example, i.e., steam cracking of naphtha. These cases, with different values for the four disquisitional parameters as shown in c.f. Table 3, are:
(i) Reference example: State-of-the-fine art electrolyzer performance based on Ogura et al. (2004) with a product selectivity of 70% and conversion of l%, CO2 value of −30 €/metric ton and an industrial electricity price of 35 €/MW h (Haegel et al., 2017).
(2) High selectivity: Selectivity of 100% and conversion of l%, CO2 value of −30 €/metric ton and an industrial electricity price of 35 €/MW h.
(3) Loftier conversion: Selectivity of lxx% and conversion of 100%, CO2 value of −30 €/metric ton and an industrial electricity price of 35 €/MW h.
(4) High CO2 cost: Selectivity of 70% and conversion of l%, CO2 value of −100 €/metric ton and an industrial electricity price of 35 €/MW h.
(5) Free electricity: Selectivity of 70% and conversion of 50%, COii value of −thirty €/metric ton and zero toll electricity.

Tabular array 3. Values for the three input parameters, i.e., production selectivity (%), conversion (%), COii value (€/metric ton) and electricity price (€/MW h) for the 5 different cases.
Results and Discussion
Economic Evaluation
In Effigy half-dozen, the main energy input and material streams considered in the economic analysis are shown. Note that for the reference instance, the loftier COtwo cost case and the complimentary electricity case, these values are identical, as the only deviation between these iii cases is caused by a change in CO2 and electricity price. For a production selectivity of 70%, a larger flow of CO2 is required to obtain the desired ethylene production chapters (10v metric ton per twelvemonth), i.e., 4.48 × 10v metric ton CO2 per year compared to 3.xiv × 105 metric ton COtwo per twelvemonth in case of a product selectivity of 100%. This lower product selectivity leads to larger energy requirements for the CO2 capture step. The thermal energy required in the CO2 capture stride is equal to approximately 87% of the full energy need. The thermal energy input in the COii conversion stride is used in the recycle loop for the separation of unconverted COtwo from the gaseous product. In the case of consummate conversion of CO2, at that place is no demand for this separation stride and hence the thermal energy input becomes negligible. The free energy consumption for the COtwo conversion stride is dominated by the electricity need. Taking into account that the plant runs for 8,440 h in one year (or 96% of the time), this corresponds to a continuous power requirement of 367 MW. If the production selectivity decreases to 70%, the required electric ability increases to 751 MW.
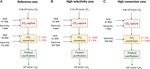
FIGURE vi. Black box representations of the electrochemical reduction of CO2 with the electrolyzer performance, i.eastward., conversion (C) and selectivity (Due south), energy input and material streams that have been considered in the economical assay for the (A) reference, high CO2 cost and free electricity cases, (B) the high selectivity case, and (C) the high conversion example.
Figure 7 summarizes the CAPEX, OPEX and gross margins, i.e., the deviation betwixt revenues and feedstock price, for the five studied cases and the base of operations instance, i.due east., naphtha-based steam cracking. It tin exist seen that currently the main disadvantage of the electrochemical process is the loftier CAPEX of the electrochemical reactor, which is a issue of expensive electrode materials combined with limited economies of scale due to the modular character of the electrochemical cells. Nevertheless, when considering the gross margin, the electrochemical process looks promising compared to the conventional steam cracking road, due to the lower feedstock cost. Hence, hereafter R&D efforts should aim to develop highly active, selective, stable and low-cost electrocatalysts in order to subtract the reactor CAPEX. For each case, the CAPEX, OPEX, and gross margin of the different steps, i.eastward., COii capture, CO2 conversion, and product separation, can be found in the Supplementary Fabric.
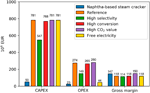
Figure 7. Capital expenditure, Operational expenditure, and gross margin (in 10six €) for the naphtha-based steam cracker, the reference electrochemical case, the high selectivity example, the loftier conversion case, the high COii cost case and the gratuitous electricity case.
Sensitivity Assay
A global sensitivity analysis has been conducted in order to investigate the influence of the dominant parameters, i.e., selectivity, conversion, CO2 cost, and electricity price, on the economics of the electrochemical process. The results for the sensitivity of the CAPEX, OPEX, and gross margin with respect to the reference electrochemical case are shown in Figure eight. As expected the selectivity to ethylene is the parameter with the largest influence on the CAPEX and the OPEX of the process. For a lower selectivity, more free energy is lost in byproduct germination. More than energy is thus required to obtain a desired production capacity of ethylene. A higher electricity consumption likewise increases the total electrolyzer area, resulting in a higher electrolyzer CAPEX. The conversion, which determines the required capacity of the recycle loop, has only a limited influence. This is too the example for the price of CO2, which is encouraging every bit information technology reduces the dependence of the process on a gene that is mainly adamant by macro-economical and political factors ("COtwo tax" vs. capture costs). The electricity price is i of the principal parameters influencing the economic feasibility of the process. Some people argue whether or non it would exist beneficial to operate an electrochemical process merely when the electricity price is below a sure threshold value, i.e., operate the product plant in a flexible manner co-ordinate to the energy market. The mild operating atmospheric condition, i.east., ambient temperature, and the modular character of a earth-calibration plant would let rapid changes in production rate. However, due to the extremely loftier CAPEX of the electrochemical process, we believe that it would exist more beneficial to run the process continuously with a fluctuating energy price, rather than operating information technology equally a discontinuous process. This is primarily motivated by the payback time and for the chemic manufacture this is typically in the order of a decade for large investments (Anderson and Fennell, 2013). Turning the installation on and off would result in an unacceptably loftier payback time. Besides, the applied feasibility of ramping upwards or down such a big calibration product unit of measurement should be considered and there is not a lot of published work on the start-up and shutdown of electrochemical reactors (Rousar et al., 1986; Bisang 1997).
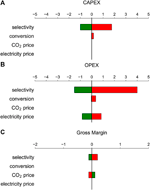
FIGURE 8. Sensitivity assay of the (A) Upper-case letter expenditure (CAPEX), (B) Operational expenditure (OPEX), and (C) gross margin for a positive (green) or negative (scarlet) modify of the model parameters (selectivity, conversion, CO2 price and electricity price) for the reference electrochemical case.
Energy Considerations
If the CO2 reduction procedure results in an overall negative net CO2 balance, it tin be considered a viable carbon recycling technology. This means that the corporeality of COtwo emitted during the consummate process needs to be lower than the amount of CO2 converted. For naphtha-based steam cracking, the CO2 emissions are approximately one metric ton CO2 per metric ton ethylene (Ren et al., 2006). These emissions are the consequence of fuel combustion and utilities, both of which apply fossil fuel. The main contributor is the furnace with over 90% of the CO2 emissions (Amghizar et al., 2020). Because the electrochemical reduction of CO2 to ethylene requires a meaning amount of electrical power, information technology is clear that the electricity needs to come from depression-carbon energy sources, such as wind and solar energy, to obtain an overall negative COii balance. Because of their intermittent nature, both solar and wind energy have a reduced capacity factor, which is non accounted for in the presented assay. We assume that access to green electricity is continuous and steady country functioning is possible. The CO2 emissions of the culling ethylene production route are based on a Life Cycle Analysis, adopting a cradle-to-gate boundary, i.e., usage and end-of-life handling are not included (von der Assen et al., 2013). In this analysis, the CO2 feedstock is considered as a regular feedstock with its own product emissions. The emission intensities for Northwestern Europe, expressed in kg CO2 equivalents per MW h for natural gas, solar and wind are equal to respectively 490, 48, and 12 kg CO2eq/MW h (Schlömer et al., 2015). In Figure 9, the CO2 emissions per metric ton of ethylene produced are compared between the base case, i.eastward., steam cracking of naphtha, and the electrochemical reduction of CO2 using gray electricity from a natural gas ability institute, and green electricity from both solar and wind free energy. The overall COtwo balance for the electrochemical road is based on an ideal electrolyzer, i.e., operating at a conversion and selectivity of 100%. The production of 1 metric ton of ethylene requires 3.xiv metric ton of COii as carbon feed, while the electrolyzer uses approximately x MW h per metric ton CO2. The thermal energy demand for the CO2 capture step, i.due east., three.2 GJ/metric ton CO2 amounts to 0.56 metric ton CO2 per metric ton ethylene. The CO2 emissions related to the conversion step are equal to i.50 and 0.37 metric ton CO2 per metric ton ethylene, using respectively solar and wind free energy. The minor deviation in emission intensity betwixt these two low-carbon energy sources leads to a significant difference in CO2 emissions per metric ton CO2 due to the large electricity demand. From Figure nine, it can exist seen that this alternative product route of ethylene tin potentially lead to a reduction of CO2 emissions. Note that the COii emissions related to the production separation and purification steps are not taken into account in this calculation.
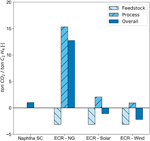
Figure nine. CO2 emissions per metric ton of ethylene produced: Comparison between steam cracking (SC) of naphtha and the electrochemical reduction (ECR) of CO2 using electricity from natural gas (NG), solar and current of air free energy. For the ECR process, both the CO2 used equally feedstock too as the COtwo emitted during the complete process is considered.
As stated earlier, it is clear that the electroreduction of CO2 needs to exist powered by green electricity in club to obtain an overall negative internet CO2 balance. The fact that reducing CO2 emissions through CCU processes will only be possible if the electricity (and in some cases the thermal energy) inputs are from renewable sources has been included in earlier studies (Bennett et al., 2014; Jouny et al., 2018; Spurgeon and Kumar, 2018; Mohsin et al., 2020). Ane of the challenges for electrolyzers powered by renewable free energy is the operation at strongly fluctuating power inputs and with frequent interruptions due to low input (Mergel et al., 2013). The dynamic behavior of the electrochemical reactor equally well as the downstream organisation components, (due east.grand., electrolyte circuit, gas separator) needs to be analyzed such that load changes do non present whatsoever problems over a large ability input range. For water electrolysis, systems have been developed which allow for a large fractional load range (v–100%) and can adapt extreme overloads. Operating the electrolyzer at a very low level (i.e., at x% of peak load) avoids the need to shut down the chemical plant completely and the associated energy losses during kickoff-upwards (Brauns and Turek, 2020). In instance the electrolyzer has been designed for constant conditions, the occurring ability fluctuations can exist damped by additional energy storage devices, which are charged when excess renewable energy is bachelor. Overall, it tin can be concluded that more theoretical and experimental piece of work is needed to better sympathize the dynamic behavior of COtwo electrolyzers powered by intermittent renewable energy.
Instead of considering the amount of CO2 emitted or avoided per metric ton ethylene, one can also look at the amount of CO2 avoided per energy unit of green electricity. In other words, what is the nigh efficient mode to reduce COtwo emissions with 1 MW h of green electricity? With an electrical energy of 1 MW h, approximately 0.032 metric ton ethylene can be produced, assuming again an ideal electrolyzer operation. This ethylene formation converts 0.ten metric ton of CO2, but also leads to 0.030 metric ton of CO2 emitted using air current ability as renewable free energy source. The production of 0.032 metric ton ethylene via the traditional steam cracking route results in approximately 0.058 metric ton of COii emitted. Thus, by producing this ethylene via the alternative electrochemical route instead of the fossil-fuel based procedure, there is a potential of lowering the emitted CO2 by 0.xiii metric ton per MW h. Using the emission intensities, 1 MW h of electricity generated from coal and natural gas, leads to respectively 0.82 and 0.49 metric ton CO2 emitted per MW h (Schlömer et al., 2015). This implies that from an energy point of view, information technology is more benign to utilize 1 MW h dark-green electricity to replace 1 MW h gray electricity than use information technology to convert CO2 electrochemically in ethylene.
A key performance indicator oft used to compare different CCU processes is the cost of CO2 avoided, i.e., the toll to avert the emission of one metric ton of CO2 relative to a reference instance. The production price for fossil ethylene considering a establish located in Europe, is dominated by the feedstock cost and equal to approximately 700 € per metric ton of ethylene. For CO2-based ethylene, the product cost is equal to 1,950 € per metric ton of ethylene, bold again an platonic electrolyzer, and an electricity toll of 35 €/MW h. This value is in agreement with the study of Jouny et al. (2018), in which they study a product cost of approximately 2000 € per metric ton of ethylene, for their "optimistic case," using the same electricity price of 35 €/MW h and a faradaic efficiency of ninety%. The internet COii emissions for the CO2 reduction process using solar and wind energy are equal to respectively −ane.08 and −two.20 metric ton CO2 per metric ton ethylene. Based on these values, the COii abstention price amounts to 602 and 391 € per metric ton CO2 avoided. Annotation that these values are significantly higher than the recent prices of the CO2 allowances envisioned by the European Emission Trading Scheme, i.east., 28 €/metric ton COii in 2030 and 43 €/metric ton CO2.
Conclusions
Electrochemical conversion of CO2 to ethylene could exist of interest to the chemical manufacture, but several breakthroughs are needed to make this competitive with the electric current state of the fine art under current marketplace atmospheric condition. Without a substantial subtract of the electricity price and large capacity increases in renewable electricity production (to become a reliable provider at continuous low prices), this culling ethylene production route seems infeasible for the chemic industry. Due to high capital costs for the electrochemical technology, it makes no sense to run these installations only in times when renewable power would be abundantly available and hence inexpensive. Turning large scale chemical processes "on" and "off" is today economically unfavorable, non even when bold that safety would exist guaranteed and an instantaneous close down would be feasible. When combined with green electricity, east.m., wind and solar, the electrochemical reduction of CO2 tin can atomic number 82 to a negative overall CO2 balance. Still, from an free energy point of view, using green electricity to supervene upon gray electricity, has a larger COtwo avoidance potential, compared to using information technology for the electrochemical production of ethylene.
Data Availability Statement
The raw data supporting the conclusions of this commodity will be made available past the authors, without undue reservation.
Author Contributions
CP: conceptualization, formal analysis, investigation, writing—original draft, visualization, writing—review and editing. MR: conceptualization, information curation, writing—review and editing. Thousand-FR: writing—review and editing. KG: conceptualization, supervision, writing—review and editing, and funding conquering.
Conflict of Interest
Writer MR was employed by the visitor Dow Benelux BV.
The remaining authors declare that the enquiry was conducted in the absence of any commercial or financial relationships that could be construed as a potential disharmonize of interest.
Funding
CP acknowledges fiscal support from a doctoral fellowship from the Enquiry Foundation—Flanders (FWO). The research leading to these results has also received funding from the European Research Council under the Eu's Horizon 2020 enquiry and innovation program (ERC Grant Agreement No. 818607).
Acknowledgments
Kees Biesheuvel, Ronald Wevers, Hanne Schatteman, Hannes Saeid Bakhsh, and Thomas Vandeputte are thanked for their valuable contributions.
Supplementary Material
The Supplementary Material for this commodity can be found online at: https://world wide web.frontiersin.org/articles/10.3389/fenrg.2020.557466/full#supplementary-material
Abbreviations
CCS, carbon capture and sequestration; CCU, carbon capture and utilization; FE, Faradaic efficiency; EE, energy efficiency; CAPEX, uppercase expenditure; OPEX, operational expenditure; MEA, monoethanolamine; HVC, high-value chemical; BTX, benzene, toluene, xylene
References
Agarwal, A. S., Zhai, Y., Loma, D., and Sridhar, N. (2011). The electrochemical reduction of carbon dioxide to formate/formic acid: engineering and economical feasibility. ChemSusChem 4, 1301–1310. doi:10.1002/cssc.201100220
CrossRef Total Text | Google Scholar
Amghizar, I., Vandewalle, 50. A., Van Geem, K. One thousand., and Marin, G. B. (2017). New trends in olefin production. Engineering science 3, 171–178. doi:x.1016/j.eng.2017.02.006
CrossRef Total Text | Google Scholar
Amghizar, I., Dedeyne, J. N., Chocolate-brown, D. J., Marin, Grand. B., and Van Geem, K. Chiliad. (2020). Sustainable innovations in steam smashing: COii neutral olefin product. React. Chem. Eng v, 239–257. doi:10.1039/c9re00398c
CrossRef Full Text | Google Scholar
Anderson, J., and Fennell, A. (2013). Calculate financial indicators to guide investments. Chem. Eng. Prog 109, 34–40.
Google Scholar
Banholzer, W. F., and Jones, M. E. (2013). Chemical engineers must focus on practical solutions. AIChE J 59, 2708–2720. doi:10.1002/aic.14172
CrossRef Full Text | Google Scholar
Banholzer, W. F. (2012). Practical limitations and recognizing hype. Free energy Environ. Sci 5, 5478–5480. doi:10.1039/c2ee03146a
CrossRef Full Text | Google Scholar
Bard, A. J., Parsons, R., and Jordan, J.International Union of Pure and Applied Chemical science (1985). Standard potentials in aqueous solution. New York, NY: Thou. Dekker.
Bennett, S. J., Schroeder, D. J., and Mccoy, S. T. (2014). Towards a Framework for discussing and assessing CO2 utilisation in a climate context. Energy Procedia 63, 7976–7992. doi:10.1016/j.egypro.2014.eleven.835
CrossRef Full Text | Google Scholar
Betts, R. A., Jones, C. D., Knight, J. R., Keeling, R. F., and Kennedy, J. J. (2016). El Niño and a record CO2 rise. Nat. Clim. Change 6, 806. doi:x.1038/nclimate3063
CrossRef Full Text | Google Scholar
Bisang, J. 1000. (1997). Modelling the startup of a continuous parallel plate electrochemical reactor. J. Appl. Electrochem 27, 379–384. doi:10.1023/a:1018401418261
CrossRef Full Text | Google Scholar
Brauns, J., and Turek, T. (2020). Alkaline water electrolysis powered by renewable energy: a review. Processes 8, 248. doi:10.3390/pr8020248
CrossRef Full Text | Google Scholar
Dominguez-Ramos, A., Singh, B., Zhang, X., Hertwich, E. G., and Irabien, A. (2015). Global warming footprint of the electrochemical reduction of carbon dioxide to formate. J. Clean. Prod 104, 148–155. doi:10.1016/j.jclepro.2013.11.046
CrossRef Total Text | Google Scholar
Endrődi, B., Bencsik, Thousand., Darvas, F., Jones, R., Rajeshwar, K., and Janáky, C. (2017). Continuous-menstruation electroreduction of carbon dioxide. Prog. Energy Combust. Sci 62, 133–154.
Google Scholar
Engelbrecht, A., Hämmerle, M., Moos, R., Fleischer, 1000., and Schmid, G. (2016). Improvement of the selectivity of the electrochemical conversion of COii to hydrocarbons using cupreous electrodes with in-situ oxidation past oxygen. Electrochim. Acta 224, 642–648. doi:10.1016/j.electacta.2016.12.059
CrossRef Full Text | Google Scholar
Gani, R., Bałdyga, J., Biscans, B., Brunazzi, E., Charpentier, J.-C., Drioli, E., et al. (2020). A multi-layered view of chemic and biochemical engineering. Chem. Eng. Res. Des 155, A133–A145. doi:ten.1016/j.cherd.2020.01.008
CrossRef Total Text | Google Scholar
Gao, J., Zhang, H., Guo, X., Luo, J., Zakeeruddin, Due south. M., Ren, D., et al. (2019). Selective C-C coupling in carbon dioxide electroreduction via efficient spillover of intermediates equally supported by operando Raman spectroscopy. J. Am. Chem. Soc 141, 18704–18714. doi:x.1021/jacs.9b07415
CrossRef Full Text | Google Scholar
Haegel, N. M., Margolis, R., Buonassisi, T., Feldman, D., Froitzheim, A., Garabedian, R., et al. (2017). Terawatt-scale photovoltaics: trajectories and challenges. Scientific discipline 356, 141–143. doi:10.1126/science.aal1288
CrossRef Full Text | Google Scholar
Hara, One thousand., Kudo, A., and Sakata, T. (1997). Electrochemical CO2 reduction on a burnished carbon electrode under high force per unit area. J. Electroanal. Chem 421, one–4. doi:10.1016/s0022-0728(96)01028-five
CrossRef Full Text | Google Scholar
Hori, Y., Kikuchi, G., and Suzuki, S. (1985). Production of Co and CH4 in electrochemical reduction of COtwo at metal electrodes in aqueous hydrogencarbonate solution Chem. Lett 14, 1695–1698. doi:10.1246/cl.1985.1695
CrossRef Total Text | Google Scholar
Hori, Y. (2008). "Electrochemical COtwo reduction on metal electrodes," in Mod aspects of electrochemistry. Editors C. Thousand. Vayenas, R. E. White and M. Due east. Gamboa-Aldeco (New York, NY: Springer New York), 89–189.
Google Scholar
Jhong, H.-R. Chiliad., Ma, S., and Kenis, P. J. (2013). Electrochemical conversion of COtwo to useful chemicals: electric current condition, remaining challenges, and time to come opportunities. Curr. Opin. Chem. Eng ii, 191–199. doi:ten.1016/j.coche.2013.03.005
CrossRef Full Text | Google Scholar
Jouny, M., Luc, W., and Jiao, F. (2018). General techno-economical analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res 57, 2165–2177. doi:x.1021/acs.iecr.7b03514
CrossRef Full Text | Google Scholar
Kaneco, S., Iiba, K., Suzuki, Due south.-K., Ohta, K., and Mizuno, T. (1999). Electrochemical reduction of carbon dioxide to hydrocarbons with loftier faradaic efficiency in LiOH/methanol. J. Phys. Chem. B 103, 7456–7460. doi:x.1021/jp990021j
CrossRef Full Text | Google Scholar
Karl, M., Wright, R. F., Berglen, T. F., and Denby, B. (2011). Worst case scenario study to assess the environmental impact of amine emissions from a COtwo capture plant. Int. J. Greenh. Gas Con 5, 439–447. doi:10.1016/j.ijggc.2010.eleven.001
CrossRef Full Text | Google Scholar
Ke, F.-S., Liu, X.-C., Wu, J., Sharma, P. P., Zhou, Z.-Y., Qiao, J., et al. (2017). Selective formation of C2 products from the electrochemical conversion of CO2 on CuO-derived copper electrodes comprised of nanoporous ribbon arrays. Catal. Today 288, 18–23. doi:10.1016/j.cattod.2016.10.001
CrossRef Total Text | Google Scholar
Kortlever, R., Shen, J., Schouten, Grand. J. P., Calle-Vallejo, F., and Koper, M. T. Chiliad. (2015). Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J. Phys. Chem. Lett half-dozen, 4073–4082. doi:10.1021/acs.jpclett.5b01559
CrossRef Total Text | Google Scholar
Kortlever, R., Peters, I., Balemans, C., Kas, R., Kwon, Y., Mul, G., et al. (2016). Palladium-golden catalyst for the electrochemical reduction of COii to C1–C5 hydrocarbons. Chem. Commun 52, 10229–10232. doi:10.1039/c6cc03717h
CrossRef Full Text | Google Scholar
Kuhl, K. P., Hatsukade, T., Cavern, Eastward. R., Abram, D. N., Kibsgaard, J., and Jaramillo, T. F. (2014). Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J. Am. Chem. Soc 136, 14107–14113. doi:ten.1021/ja505791r
CrossRef Full Text | Google Scholar
Kuramochi, T., Ramírez, A., Turkenburg, Westward., and Faaij, A. (2011). Techno-economic assessment and comparison of CO2 capture technologies for industrial processes: preliminary results for the iron and steel sector. Free energy Procedia iv, 1981–1988. doi:10.1016/j.egypro.2011.02.079
CrossRef Full Text | Google Scholar
Leung, D. Y. C., Caramanna, Chiliad., and Maroto-Valer, K. M. (2014). An overview of current condition of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev 39, 426–443. doi:10.1016/j.rser.2014.07.093
CrossRef Full Text | Google Scholar
Li, Thou., Cousins, A., Yu, H., Feron, P., Tade, M., Luo, Westward., et al. (2016). Systematic report of aqueous monoethanolamine-based CO2 capture process: model development and process improvement. Energy Sci. Eng 4, 23–39. doi:10.1002/ese3.101
CrossRef Full Text | Google Scholar
Ma, S., Sadakiyo, Yard., Luo, R., Heima, M., Yamauchi, M., and Kenis, P. J. A. (2016). One-step electrosynthesis of ethylene and ethanol from COtwo in an alkaline electrolyzer. J. Power Sources 301, 219–228. doi:10.1016/j.jpowsour.2015.09.124
CrossRef Full Text | Google Scholar
Mao, X., and Hatton, T. A. (2015). Recent advances in electrocatalytic reduction of carbon dioxide using metal-gratis catalysts. Ind. Eng. Chem. Res 54, 4033–4042. doi:10.1021/ie504336h
CrossRef Full Text | Google Scholar
Martín, A. J., Larrazábal, Yard. O., and Pérez-Ramírez, J. (2015). Towards sustainable fuels and chemicals through the electrochemical reduction of CO2: lessons from water electrolysis. Light-green Chem 17, 5114–5130. doi:x.1039/c5gc01893e
CrossRef Total Text | Google Scholar
Mergel, J., Carmo, M., and Fritz, D. (2013). "Status on technologies for hydrogen production by water electrolysis," in Transition to renewable energy systems. (Weinheim, Germany: Wiley‐VCH Verlag GmbH & Co. KGaA), 423–450.
Google Scholar
Mistry, H., Varela, A. S., Bonifacio, C. S., Zegkinoglou, I., Sinev, I., Choi, Y.-W., et al. (2016). Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat. Commun 7, 12123. doi:ten.1038/ncomms12123
CrossRef Full Text | Google Scholar
Mohsin, I., Al-Attas, T. A., Sumon, Thou. Z., Bergerson, J., Mccoy, S., and Kibria, Chiliad. 1000. (2020). Economical and environmental cess of integrated carbon capture and utilization. Cell Rep. Phys. Sci 1, 100104. doi:x.1016/j.xcrp.2020.100104
CrossRef Full Text | Google Scholar
Ogura, K., Yano, H., and Tanaka, T. (2004). Selective formation of ethylene from COii by catalytic electrolysis at a three-phase interface. Catal. Today 98, 515–521. doi:10.1016/j.cattod.2004.09.006
CrossRef Full Text | Google Scholar
Oloman, C., and Li, H. (2008). Electrochemical processing of carbon dioxide. ChemSusChem 1, 385–391. doi:ten.1002/cssc.200800015
CrossRef Full Text | Google Scholar
Brownnose, Iii, J. E., Ren, D., and Yeo, B. S. (2017). Practices for the collection and reporting of electrocatalytic operation and mechanistic information for the CO2 reduction reaction. Catal. Sci. Technol vii, 5820–5832. doi:10.1039/c7cy01785e
CrossRef Total Text | Google Scholar
Peng, Y., Wu, T., Sun, L., Nsanzimana, J. M. V., Fisher, A. C., and Wang, X. (2017). Selective electrochemical reduction of CO2 to ethylene on nanopores-modified https://www.spglobal.com/platts/en/our-methodology/toll-assessments/petrochemicals/pgpi-propylene copper electrodes in aqueous solution. ACS Appl. Mater. Interfaces 9, 32782–32789. doi:x.1021/acsami.7b10421
CrossRef Full Text | Google Scholar
Peterson, A. A., and Nørskov, J. M. (2012). Activeness descriptors for CO2 electroreduction to methane on transition-metal catalysts. J. Phys. Chem. Lett 3, 251–258. doi:10.1021/jz201461p
CrossRef Total Text | Google Scholar
Qiao, J., Liu, Y., Hong, F., and Zhang, J. (2014). A review of catalysts for the electroreduction of carbon dioxide to produce depression-carbon fuels. Chem. Soc. Rev 43, 631–675. doi:10.1039/c3cs60323g
CrossRef Full Text | Google Scholar
Rakowski Dubois, M., and Dubois, D. L. (2009). Development of molecular electrocatalysts for CO2 reduction and H2 production/oxidation. Acc. Chem. Res 42, 1974–1982. doi:10.1021/ar900110c
CrossRef Full Text | Google Scholar
Ren, T., Patel, M., and Blok, Chiliad. (2006). Olefins from conventional and heavy feedstocks: energy use in steam bully and alternative processes. Free energy 31, 425–451. doi:x.1016/j.energy.2005.04.001
CrossRef Full Text | Google Scholar
Ren, T., Patel, M. Grand., and Blok, K. (2008). Steam bully and methane to olefins: free energy use, CO2 emissions and production costs. Energy 33, 817–833.
Google Scholar
Rockström, J., Gaffney, O., Rogelj, J., Meinshausen, Yard., Nakicenovic, N., and Schellnhuber, H. J. (2017). A roadmap for rapid decarbonization. Science 355, 1269–1271. 10.1126/science.aah3443
CrossRef Full Text | Google Scholar
Rousar, I., Micka, Thousand., and Kimla, A. (1986). Electrochemical engineering. Amsterdam, Netherlands: Elsevier.
Google Scholar
Schlömer, S., Bruckner, T., Fulton, L., Hertwich, E., Mckinnon, A., Perczyk, D., et al. (2015). "Technology-specific price and performance parameters," in Climate change 2014: mitigation of climate change: working group III contribution to the IPCC 5th assessment report. Editor Schlömer, S. (Cambridge, United kingdom of great britain and northern ireland: Cambridge Academy Printing), 1329–1356.
Google Scholar
Schneider, J., Jia, H., Muckerman, J. T., and Fujita, E. (2012). Thermodynamics and kinetics of CO2, CO, and H+ binding to the metal center of CO2 reductioncatalysts. Chem. Soc. Rev 41, 2036–2051. doi:10.1039/c1cs15278e
CrossRef Full Text | Google Scholar
Schouten, K. J. P., Calle-Vallejo, F., and Koper, M. T. M. (2014). A step closer to the electrochemical production of liquid fuels. Angew. Chem. Int. Ed 53, 10858–10860. doi:x.1002/anie.201406174
CrossRef Full Text | Google Scholar
Spigarelli, B. P., and Kawatra, S. Thousand. (2013). Opportunities and challenges in carbon dioxide capture. J. CO2 Util one, 69–87. doi:ten.1016/j.jcou.2013.03.002
CrossRef Total Text | Google Scholar
Spurgeon, J. M., and Kumar, B. (2018). A comparative technoeconomic analysis of pathways for commercial electrochemical CO2 reduction to liquid products. Energy Environ. Sci eleven, 1536–1551. doi:10.1039/c8ee00097b
CrossRef Full Text | Google Scholar
Tao, M., Qun, F., Hengcong, T., Zishan, H., Mingwen, J., Yunnan, G., et al. (2017). Heterogeneous electrochemical CO2 reduction using nonmetallic carbon-based catalysts: current status and futurity challenges. Nanotechnology 28, 472001.
Google Scholar
Van Geem, Thousand. M., Galvita, 5. V., and Marin, G. B. (2019). Making chemicals with electricity. Science 364, 734. doi:x.1126/scientific discipline.aax5179
CrossRef Total Text | Google Scholar
Voltachem (2016). Empowering the chemical industry: opportunities for electrification. Den Haag, Netherlands: TNO.
Google Scholar
Von Der Assen, Northward., Jung, J., and Bardow, A. (2013). Life-cycle assessment of carbon dioxide capture and utilization: avoiding the pitfalls. Free energy Environ. Sci half dozen, 2721–2734. doi:10.1039/c3ee41151f
CrossRef Full Text | Google Scholar
Whipple, D. T., and Kenis, P. J. A. (2010). Prospects of COii Utilization via direct heterogeneous electrochemical reduction. J. Phys. Chem. Lett 1, 3451–3458. doi:10.1021/jz1012627
CrossRef Full Text | Google Scholar
Wu, J., Ma, S., Lord's day, J., Gold, J. I., Tiwary, C., Kim, B., et al. (2016). A metal-free electrocatalyst for carbon dioxide reduction to multi-carbon hydrocarbons and oxygenates. Nat. Commun 7, 13869. doi:10.1038/ncomms13869
CrossRef Total Text | Google Scholar
Zhai, Y., Chiachiarelli, L., and Sridhar, N. (2019). Outcome of gaseous impurities on the electrochemical reduction of COtwo on copper electrodes. ECS Trans nineteen, i–13. doi:10.1149/ane.3220175
CrossRef Full Text | Google Scholar
Zimmermann, H., and Walzl, R. (2000). "Ethylene," in Ullmann's Encyclopedia of Industrial Chemistry. (Wiley-VCH Verlag GmbH & Co. KGaA).
CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fenrg.2020.557466/full
Belum ada Komentar untuk "A Review of Catalysts for the Electroreduction of Carbon Dioxide to Produce Low-carbon Fuels"
Posting Komentar